A system of quality management, built to operate day to day
Quality management standards now require active, ongoing systems, not static annual documentation. Caseware supports this shift with a connected, standards-aligned environment that links objectives, risks, responses and evaluation into everyday firm operations.

What firms achieve with Caseware

Confidence across global standards
Operate a unified quality management system aligned to ISQM, SQMS, CSQM, QC 1000, and related frameworks.

From policy to practice
Move beyond static manuals by linking quality objectives and risks directly to responses, tasks and accountability.

Ongoing oversight, not annual scramble
Continuously monitor activity, completion and effectiveness instead of preparing retrospectively for inspections.

Scalability as the firm grows
Support different roles, responsibilities and structures across offices, service lines and networks without losing consistency.
A proactive approach to quality management
Quality management is no longer a once-a-year exercise. It is an ongoing cycle that must adapt as risks, people and engagements change.
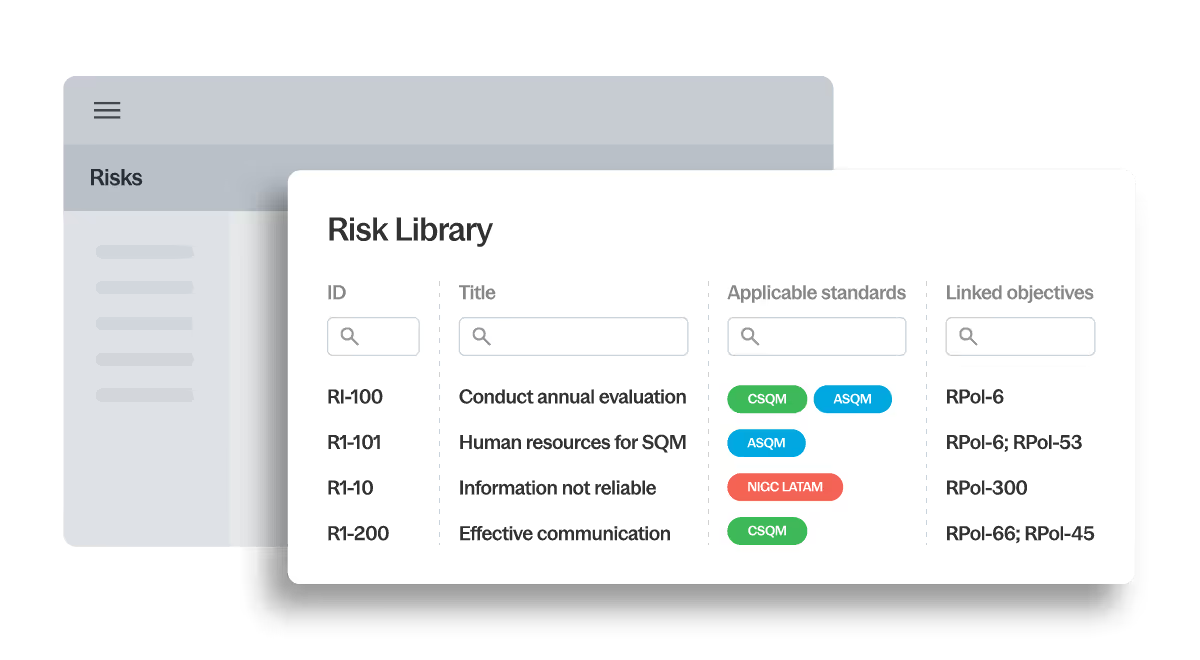
Define quality objectives, identify risks and link responses using guided workflows mapped directly to global quality standards. Built-in content libraries help firms start with trusted structures rather than blank documents.
- ISQM, SQMS, CSQM, QC 1000 alignment
- Structured quality objectives
- Linked risks and responses
- Consistent global design
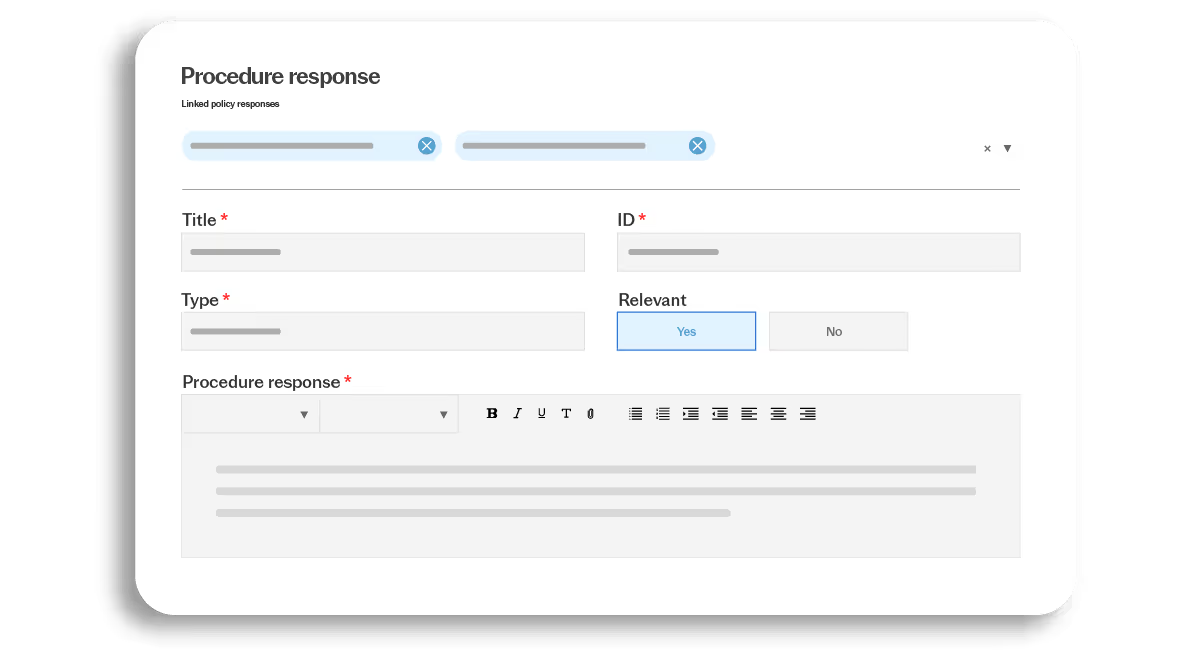
Translate quality responses into assigned tasks with clear ownership and timelines. Responsibilities remain visible even as roles change, helping ensure quality requirements are executed in practice.
- Role-based task assignments
- Clear accountability
- Ongoing execution tracking
- Reduced reliance on reminders
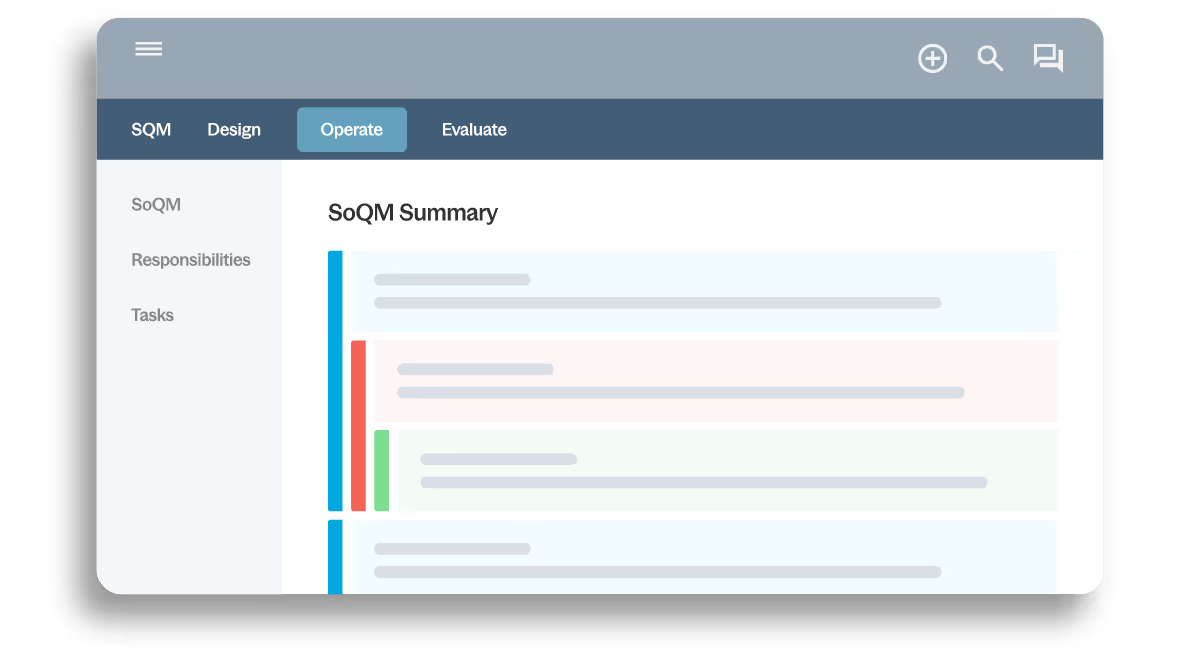
Maintain a complete, accessible record of objectives, risks, responses, policies, tasks and sign-offs in one system. Automatic linkages and version history support transparency and inspection readiness.
- Centralized quality documentation
- Version control and history
- Electronic sign-offs
- Defensible audit trail
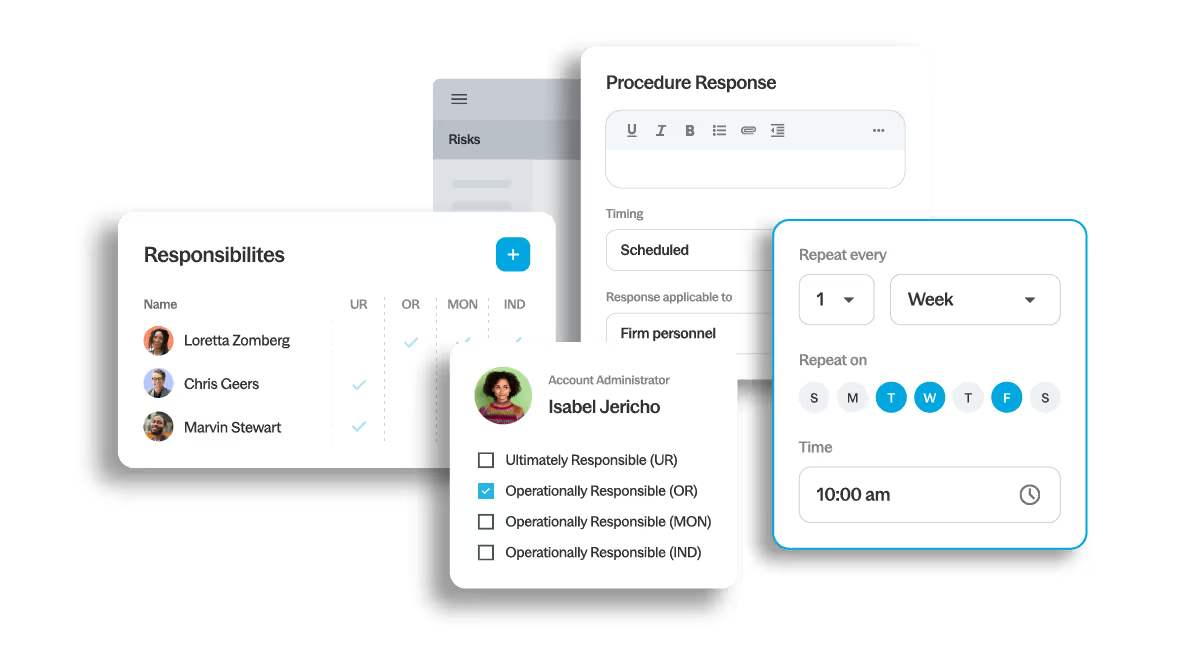
Track activity, assess effectiveness and document evaluations using dashboards and linked reports. When issues arise, root causes and remediation actions are captured and followed through.
- Real-time monitoring
- Structured evaluations
- Root-cause analysis
- Documented remediation

Related products
Frequently Asked Questions
The solution supports the design, operation, monitoring, and evaluation of a system of quality management aligned to global standards such as ISQM, SQMS, CSQM and QC 1000. Objectives, risks and responses are structured to reflect the intent of these standards rather than static compliance checklists.
Quality objectives, risks, responses and tasks are linked and actively managed. Responsibilities are assigned, progress is tracked and evidence is collected continuously, turning quality management into an operational process rather than a document.
The solution supports ongoing monitoring through dashboards and structured reporting. Firms can evaluate the effectiveness of their system, document findings, identify root causes and track remediation actions as part of a continuous cycle.
Yes. The system supports role-based access, multiple regions and network-defined content while allowing local adaptation. This enables firms to maintain global consistency without enforcing a one-size-fits-all approach.
Quality management is typically shared across leadership, partners and designated operational roles. The system supports this by assigning responsibilities, tracking completion and maintaining visibility so quality ownership does not sit with a single individual or become dependent on institutional knowledge.




